FDA Recalls Zimmer Biomet Shoulder Replacement
Posted on - Thursday, February 16, 2017 under Dangerous Drugs & Products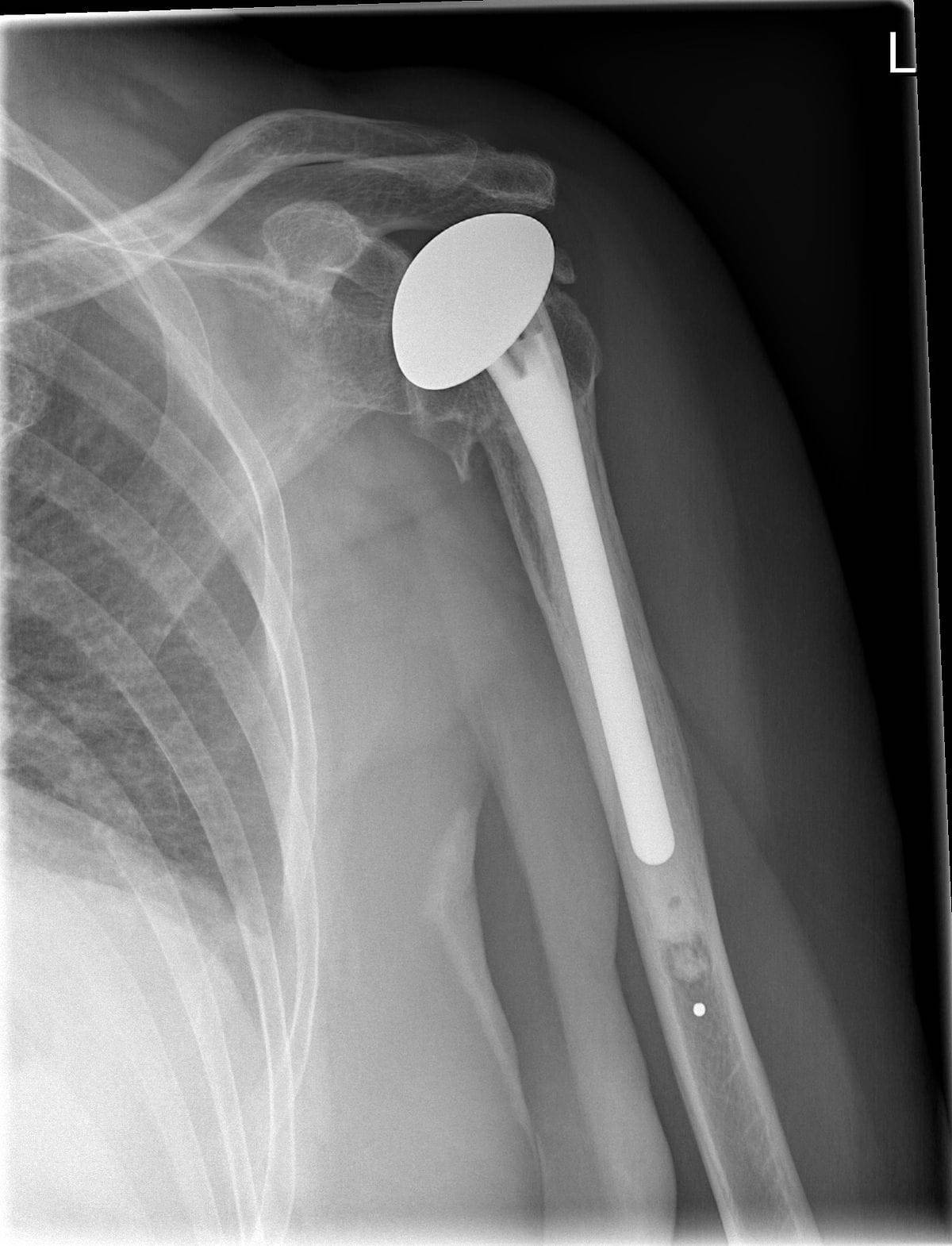
The Food & Drug Administration issued a Class 1 recall on the Zimmer Biomet Comprehensive Reverse Shoulder System Humeral Tray Model 115340. A type 1 recall is the worst kind issued by the FDA.
On the market between October of 2008 and September of 2015, it is estimated that over 3,500 people are affected by this recall.
When released, this implant was considered one of a kind. The device showed remarkable effectiveness in patients with severe shoulder arthritis in which prior joint replacement was ineffective.
Zimmer Biomet sent out an SOS signal (formally known as an Urgent Medical Device Recall Notice) on December 20, 2016 to all known affected customers.
Atlanta Shoulder Replacement Attorneys
If you believe you or a loved one has one of these recalled shoulder joint replacements, call our experienced Atlanta shoulder replacement recall Attorneys at (770) 727-5550 to discuss today.








